
Atom Modeli Lityum ( Bohr Atom Modeli ) YouTube
1 Ry = e4me 8ϵ2 0h2 = 2.18 × 10 − 18 J. and this simplifies the allowed energies predicted by the Bohr model (Equation 7.4.11) as. En = − (2.18 × 10 − 18)Z2 n2 J = − Z2 n2 Ry. Hence, the energy of the electron in an atom also is quantized. Equation 7.4.12 gives the energies of the electronic states of the hydrogen atom.
ATOM MODELİ YAPILIŞI.Basit Atom Modeli yapımı.Atom modeli nasıl yapılır?Rutherford,Bohr. YouTube
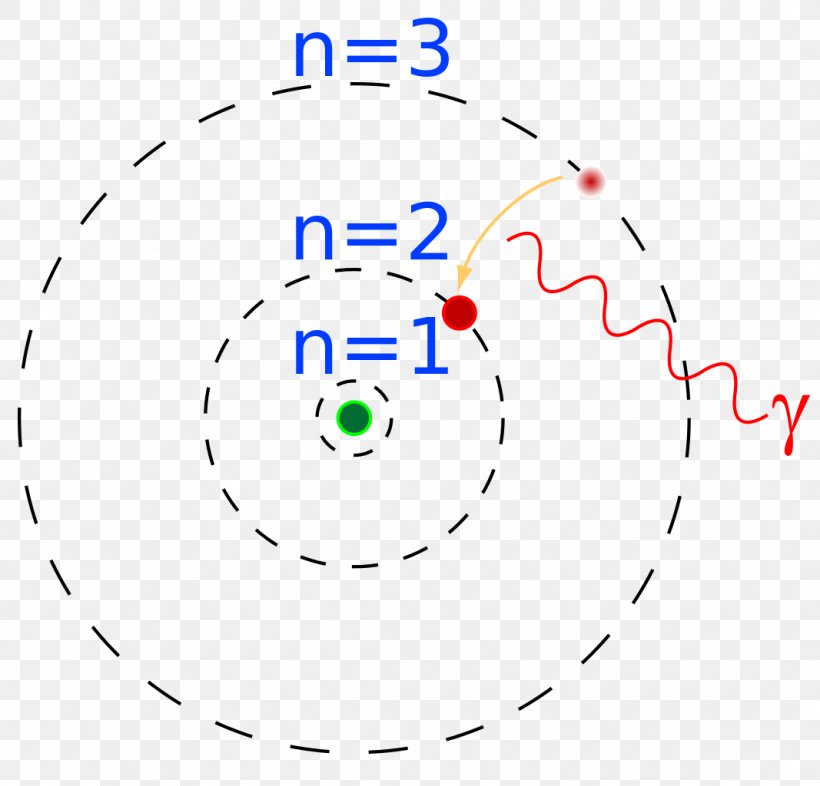
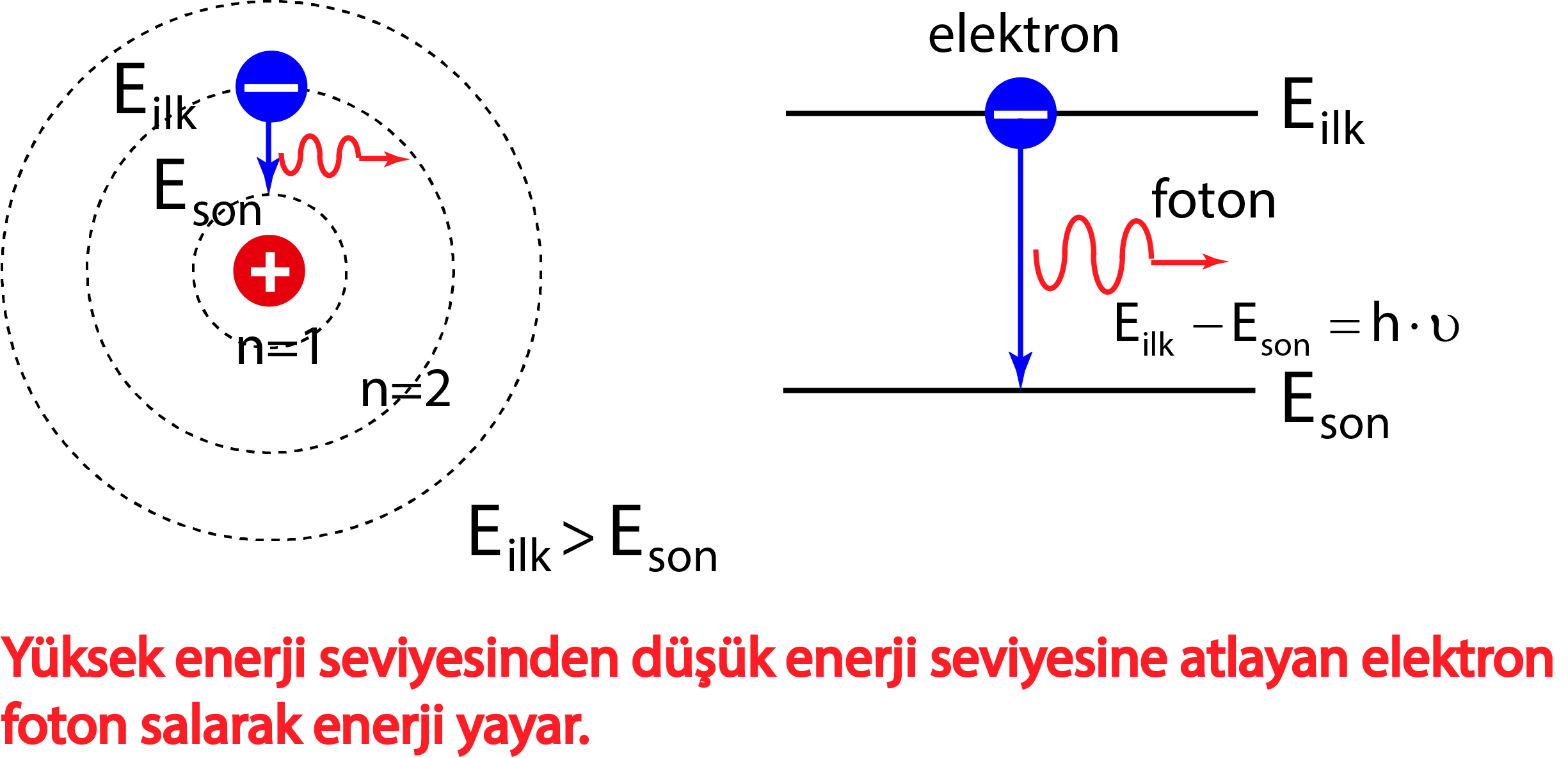
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.
Bohr Model Atomic Theory Model Atomic Free Electron Model, PNG, 1067x1024px, Bohr Model, Area
Examples on Bohr Atomic Model. Example 1: Calculate the maximum number of electrons an o shell can hold. Solution: We know that O shell means 5th shell. Therefore, n=5. Applying the formula 2n 2 = 2 x 5 2 = 50. Thus, the maximum number of electrons O shell can hold is 50.

Bohr's Atomic Model Postulates Diagram Limitations
Rutherford explained the nucleus of an atom and Bohr modified that model into electrons and their energy levels. Bohr's model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. Bohr found that an electron located away from the nucleus has more energy, and the electron which.

Aluminum Bohr Model Diagram, Using The Main Group Elements Of The Periodic Table To Draw Bohr
ATOM MODELİ YAPILIŞI.Basit Atom Modeli yapımı.Atom modeli nasıl yapılır?Rutherford,Bohr.

Atom Modeli YouTube
Bohr's Atomic Model. Following the discoveries of hydrogen emission spectra and the photoelectric effect, the Danish physicist Niels Bohr (1885-1962) proposed a new model of the atom in 1915. Bohr proposed that electrons do not radiate energy as they orbit the nucleus, but exist in states of constant energy that he called stationary states.

konuşkan Sıçrama karar bohr modeli
Resources. Lecture Slides (PDF - 9.3MB) Periodic Table and Table of Constants. Lecture Summary. Prof. Sadoway talks about the principles of modern chemistry and how that led to the understanding of the structure of the atom.He details Bohr's postulates for the hydrogen atom and discusses how the Planck-Einstein relationship applies to electron transitions.
mühendislik üçlü Titreme dalton atom modeli şekli
Bohr Atom Modoli Modern Atom Teorisi (Bulut Modeli) Şimdi bu modelleri sıraysıyla görelim. Bu bir reklamdır: Dalton Atom Modeli (1803) John Dalton John Dalton (1766-1844) yaptığı çalışmalar sonucunda atomu şöyle tarif etmiştir: Maddeler atomlardan oluşur. (Doğru)
Bohr Atom Modeli
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.
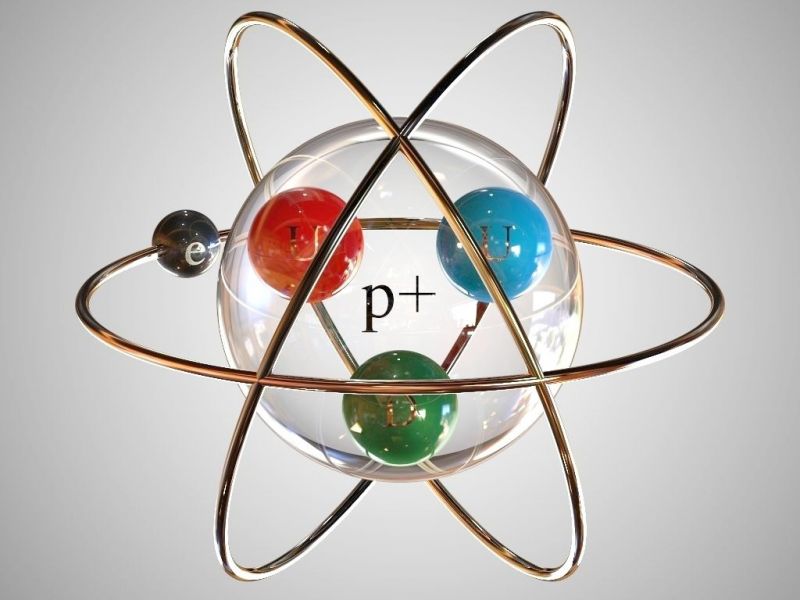
3 boyutlu hidrojen atom modeli
In 1913, Danish physicist Niels Bohr applied Max Planck's quantum theory to the nuclear atom of Ernest Rutherford, thus formulating the well-known planetary model of the atom, wherein electrons orbit a central nucleus in well-defined levels of energy ().Note that Bohr stated that electrons in the atom follow elliptical orbits (not circles as is often pictured).
:max_bytes(150000):strip_icc()/atomic-structure-conceptual-artwork-99312661-58af58c75f9b5860467ff472.jpg)
History of Atomic Theory
Strafor köpük çöp şiş bakır tel ve alüminyum folyo kullanarak Bohr lityum Atom Modeli oluşturduk
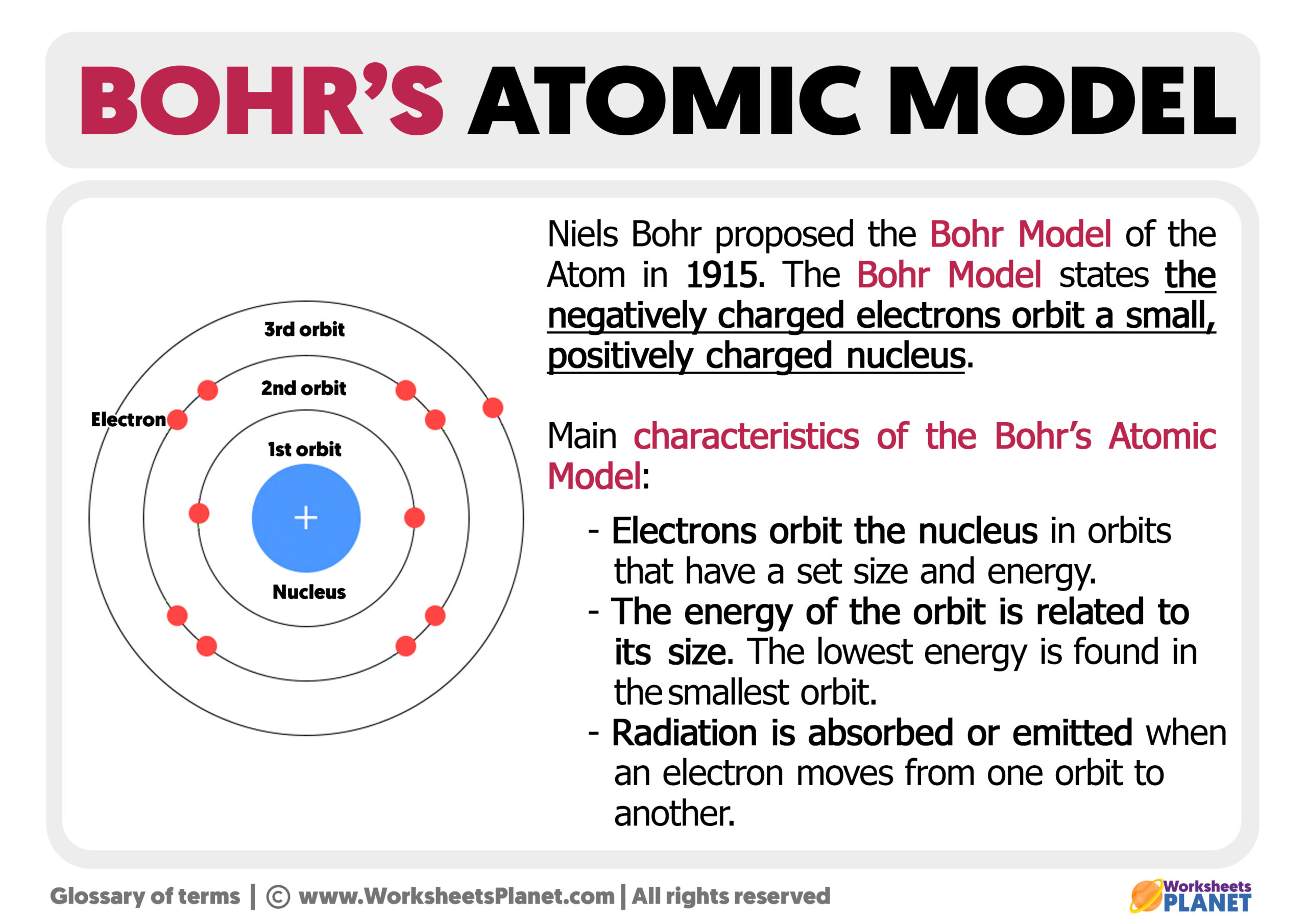
Bohr's Atomic Model
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.

Atom Modelleri(Dalton, thomson, rutherford, bohr atom modelleri) YouTube
He was struggling to make sense of all of this. As was common with Bohr when confronted with a puzzle, this struggle was nearly all-consuming. Then in 1913 Bohr, by accident, stumbled across Balmer's numerology for the hydrogen spectrum, and in a flash came up with a workable model of the atom. The model asserts that: The planetary model is.
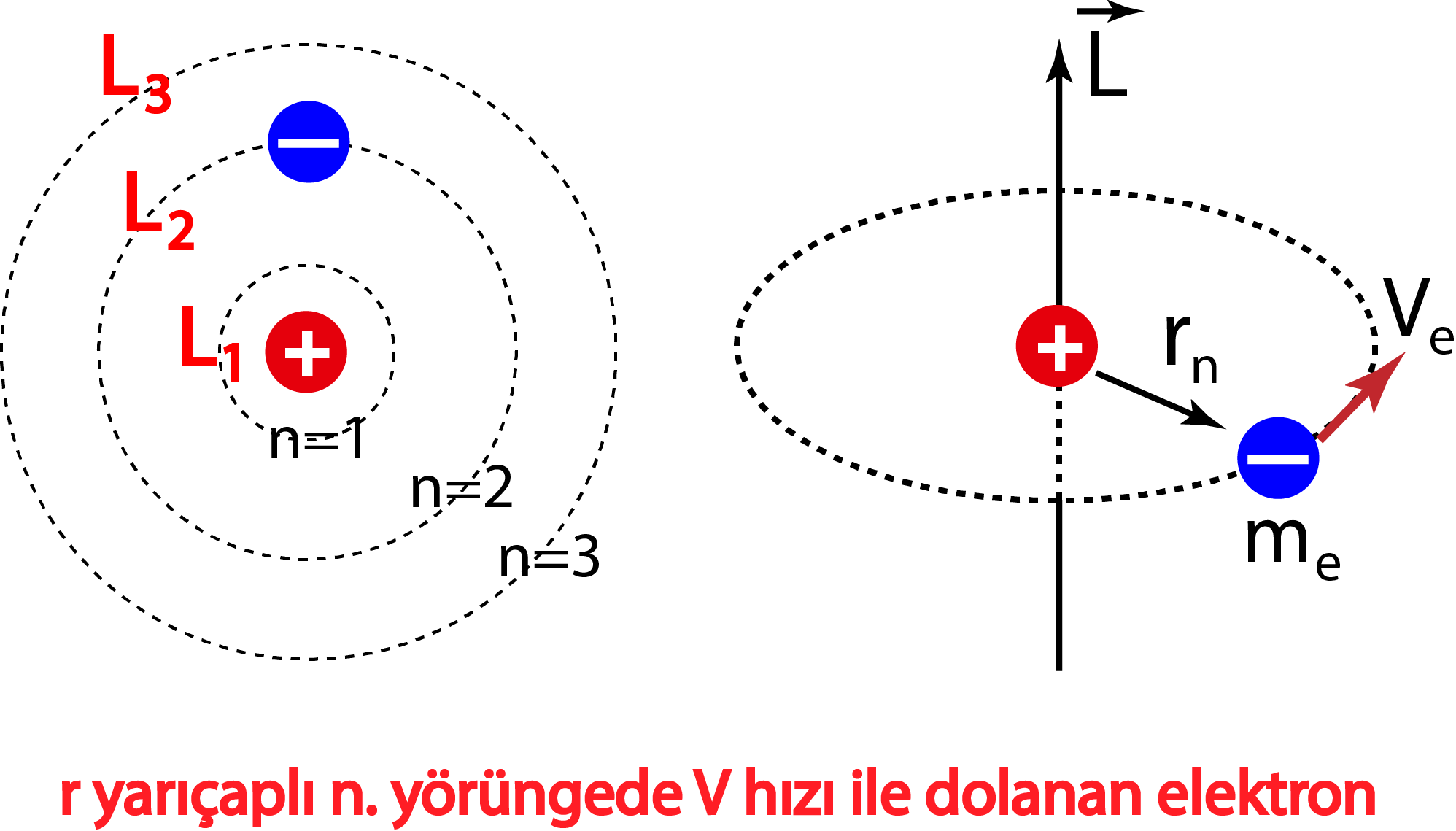
Bohr Atom Modeli Fizik. Net. Tr
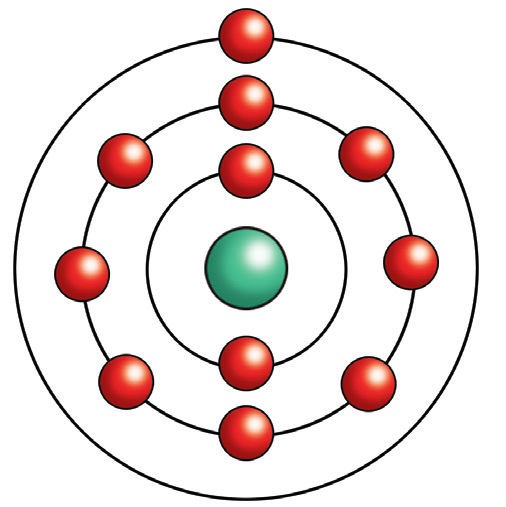
A model of a helium atom shows a black circle which fades to white moving from the center to the outside. At the center of the circle is a tiny nucleus, consisting of two red circles and two purple circles.. A Bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. The ring closest to the nucleus is labelled n=1.

Bohr's model of the hydrogen atom was the first to incorporate quantum theory, and the key idea of his model was that electrons occupy discrete orbitals. Main Idea A visualization of the Bohr model and the hydrogen spectrum. The Bohr model of the atom was proposed by Niels Bohr in 1913 as an expansion on and correction of the Rutherford model.
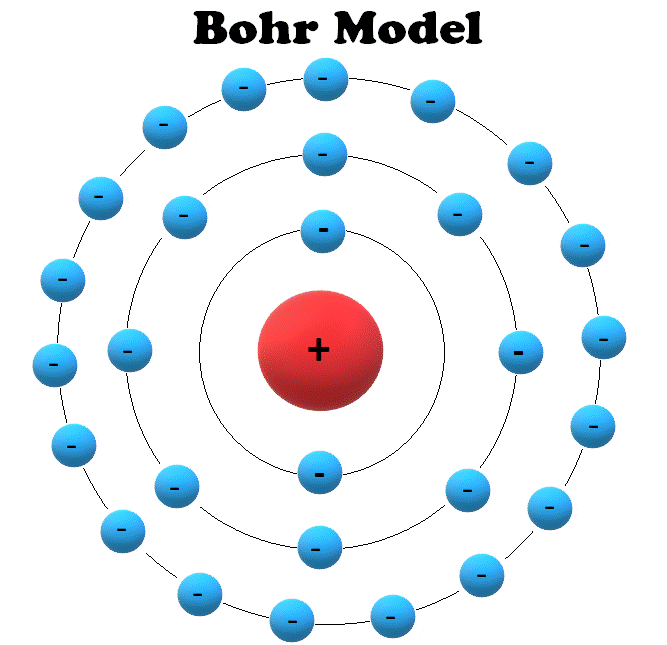
Atoms and Electrons Electronics Reference
How does Niels Bohr's atomic model work? An overview of Niels Bohr's refinement of the Rutherford model. See all videos for this article Bohr model of the atom In the Bohr model of the atom, electrons travel in defined circular orbits around the nucleus. The orbits are labeled by an integer, the quantum number n.